The Study Centre
The team at the EB Study Centre is intensively involved in determining the efficacy, tolerability and safety of new therapeutic approaches for EB. These complex tests are aimed at obtaining marketing authorisation for an effective and safe new therapy.
Clinical trials provide patients with access to innovative treatment options even before they are approved, and the trials also ensure medical progress in a variety of ways. A team of physicians, nurses and study coordinators is required to carry out clinical trials. This team is responsible for the scientific evaluation of the relevance of the study, the medical care of the patients and the regulation of and compliance with organisational procedures as well as all regulatory and legal requirements.
Professionally-sound, solidly-structured and compassionate patient care during the entire study period is also extremely important. Patients who agree to participate in a study and to become part of a new development should feel well cared for and well informed about processes that are relevant to the study.
The EB House Austria carries out both in-house academic studies (implementing in-house research results) and sponsored studies (where we are approached by pharmaceutical companies).
Carrying out clinical trials
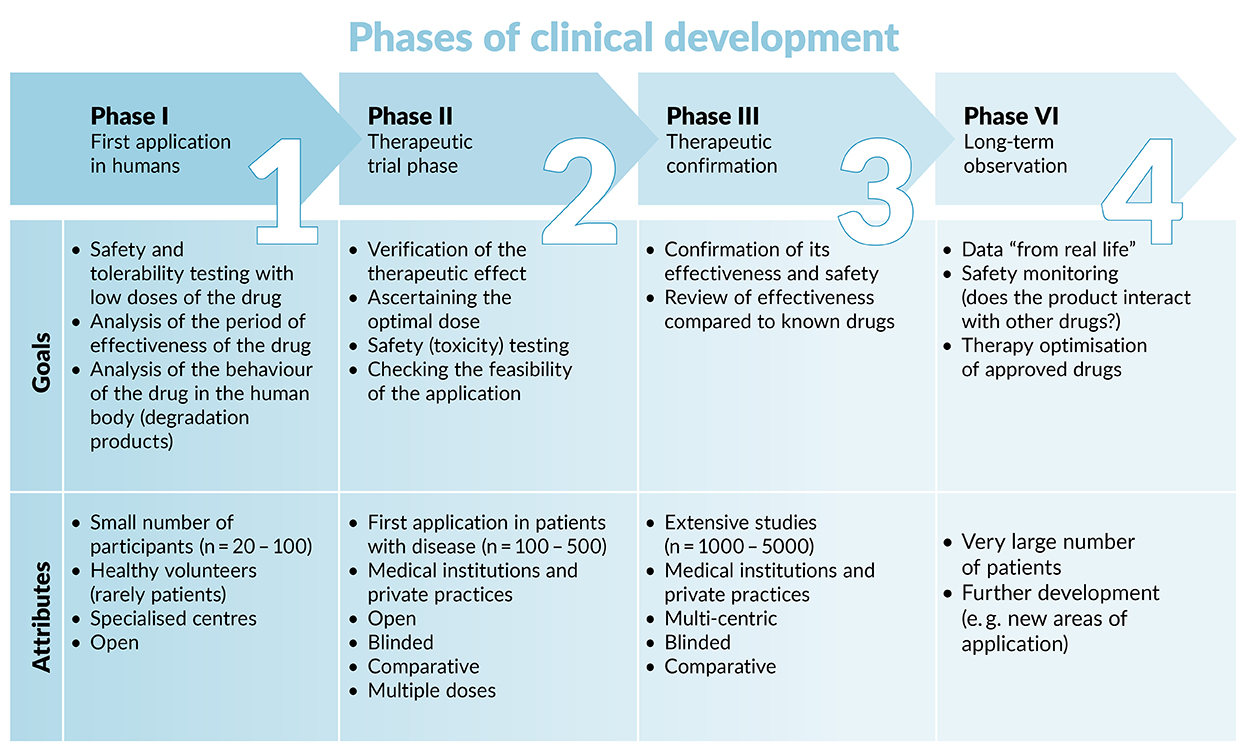
The clinical testing of medicinal products typically involves three phases (see diagram below). Since EB is a rare disease, however, and consequently not enough patients can participate in all phases, phases I + II may often be combined.
The starting point for an examination on patients is always well-founded, scientific preliminary work in the laboratory. The results obtained there must be summarised and a detailed list of the planned study (study protocol) drawn up. The list is submitted to the authorities and the ethics committees for assessment, together with the application forms, patient consent forms and qualification documents for all the persons involved. Implementation is only possible after these documents have all been approved.
The selection of EB patients is made according to strict guidelines (inclusion and exclusion criteria), which are specified in the study protocol. Due to our close contact with our patients and the (often) very detailed knowledge of their medical history, a pre-selection can be made by the study team. Patients whose profiles are suitable for participation will then be contacted. The ultimate decision for or against participation, however, always lies with the patient.
Accurate documentation is a prerequisite during the clinical trial. It facilitates the evaluation of the study data received – but the documentation must comply with official requirements. Compliance with all procedures and tests specified in the study protocol is regularly checked by the staff of an external CRO (Contract Research Organisation).
After completion of the clinical trial, the data is evaluated statistically, published in specialist journals (if possible) and the subsequent procedure is evaluated (repetition of the trial with modified parameters; a further trial to confirm effectiveness; the pursuit of marketing authorisation).